Choosing the Right Medical PCB Manufacturer: A Comprehensive Guide
In the intricate world of medical devices, the reliability of a Printed Circuit Board (PCB) is paramount. Like the human nervous system that dictates our bodily functions, the PCB acts as the central nervous system of medical equipment. Choosing the right medical PCB manufacturer is akin to selecting the finest surgeon; it's about trust, precision, and guaranteed performance. This article will dissect the key considerations when choosing a medical PCB manufacturer, ensuring your medical devices operate flawlessly and safely, contributing to advancements in healthcare and patient well-being. The role of [medical pcb manufacturer] is to ensure consistent quality, and that they are essential to the medical field's consistent and ongoing evolution.
Understanding the Importance of Medical Grade PCBs

Medical grade PCBs are distinct from standard PCBs due to their rigorous adherence to stringent quality and safety regulations, and they are paramount in ensuring the reliability and efficacy of medical devices. The selection of a specialized medical PCB manufacturer is crucial, primarily because these manufacturers are equipped to comply with the necessary standards, most notably ISO 13485, which sets the bar for quality management systems in medical device manufacturing, along with other requirements for biocompatibility, sterilization and overall product safety, which directly impacts patient safety and device functionality.
Standard PCBs, typically used in consumer electronics, lack the stringent requirements for quality management and manufacturing processes necessary for medical applications. Medical PCBs must be built to the highest standards to ensure reliable performance, even in harsh environments. They are often subjected to sterilization and must withstand high temperatures, humidity, and repetitive use.
Key Certifications and Standards for Medical PCB Manufacturers

Medical PCB manufacturers must adhere to stringent certifications and standards to ensure the safety, reliability, and efficacy of medical devices. These certifications not only demonstrate a commitment to quality but are often legally required for medical devices to be sold and used. The core certifications include ISO 13485, FDA registration, and compliance with IPC standards, along with UL and IEC requirements.
| Certification/Standard | Description | Significance |
|---|---|---|
| ISO 13485 | Quality Management System for Medical Devices. This standard specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. | Ensures consistent quality, safety, and efficacy in the design, development, production, and servicing of medical devices. |
| FDA Registration | Manufacturers of medical devices (including PCBs that are components) must register their facilities with the Food and Drug Administration (FDA) in the United States if they intend to sell products there. | Legal requirement for market access in the USA. Demonstrates compliance with FDA regulations and allows traceability of devices. |
| IPC Standards | A set of industry standards for the manufacturing, assembly, and inspection of PCBs. These standards cover various aspects like design, materials, performance, and reliability. The most relevant include IPC-A-600 (Acceptability of Printed Boards) and IPC-6012 (Qualification and Performance Specification for Rigid Printed Boards). | Guarantees consistent and high-quality PCB fabrication and assembly, improving reliability and performance. |
| UL Compliance | Underwriters Laboratories (UL) standards often involve product safety testing for electrical components and systems. Specific standards relevant to PCBs ensure electrical safety and prevent hazards. | Confirms safety aspects of PCB, particularly when used in life-supporting medical devices. Often required for product certification. |
| IEC Standards | International Electrotechnical Commission (IEC) standards, like IEC 60601-1 (Medical electrical equipment), govern the safety and performance of medical electrical equipment. PCB manufacturers need to be aware of these to supply components that do not compromise the final product's safety. | Ensures global standards for the safe performance of medical electrical equipment, including the PCBs integrated into them. |
Material Selection for Medical PCBs
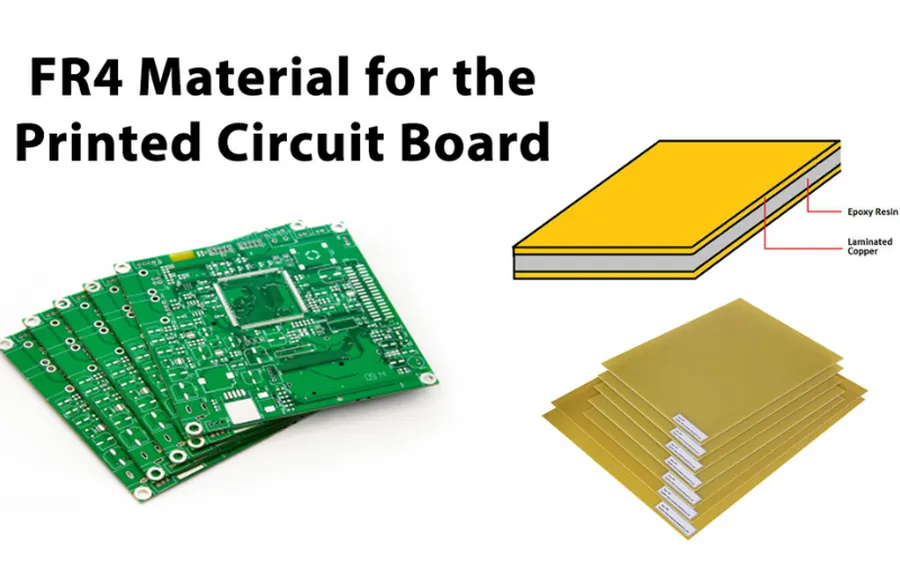
The selection of materials for medical PCBs is paramount, directly influencing the reliability, safety, and performance of medical devices. These materials must exhibit exceptional characteristics, including biocompatibility, resistance to sterilization methods, and the ability to maintain structural and electrical integrity over extended use.
| Material | Description | Typical Applications | Advantages | Considerations |
|---|---|---|---|---|
| FR-4 | A composite material composed of woven fiberglass cloth with an epoxy resin binder. | General medical devices, less critical applications. | Cost-effective, good electrical properties, widely available. | May not be suitable for high-temperature sterilization, lower biocompatibility than other options. |
| Polyimide | A high-temperature polymer known for its excellent thermal and chemical stability. | Implantable devices, high-reliability medical electronics. | Excellent thermal stability, high biocompatibility, good chemical resistance. | More expensive than FR-4, can be more challenging to process. |
| Rogers Materials | Specialized high-frequency laminates for advanced medical imaging and sensing. | High-frequency circuits, medical imaging equipment. | Superior electrical performance at high frequencies, low signal loss. | Higher cost, may require specialized manufacturing processes. |
| Teflon (PTFE) | A synthetic fluoropolymer with exceptional dielectric and chemical resistance. | Advanced medical sensors, applications requiring low signal loss. | Excellent electrical properties, very low dielectric loss, chemically inert. | Difficult to process, expensive. |
The choice of material must also consider the specific sterilization methods the PCB will undergo, such as autoclaving, ethylene oxide (EtO) sterilization, or chemical sterilization. Each method imposes different stresses on the materials, and material selection should account for these factors to prevent degradation or failure. Biocompatibility is also crucial, particularly for implantable devices. Materials that come into direct contact with human tissue must be inert and non-toxic to prevent adverse reactions. Furthermore, material selection directly affects the long-term performance and reliability of medical devices. Selecting materials that can withstand the intended use environment ensures that the medical device functions as expected throughout its service life. This includes considering thermal, mechanical, and chemical stressors.
Design Considerations for Medical PCBs

Medical PCB design demands meticulous attention to detail, going beyond typical electronic applications. Critical factors include maintaining signal integrity, carefully controlling impedance, and selecting components that meet stringent medical-grade standards. Close collaboration with your medical PCB manufacturer during the design phase is essential for optimal performance and reliability.
- Signal Integrity:
Maintaining signal integrity is paramount in medical devices, where accurate data transmission is crucial. This involves minimizing signal reflections, cross-talk, and electromagnetic interference (EMI) through careful routing, trace impedance control, and proper grounding techniques. - Impedance Control:
Precise impedance control is required, especially for high-speed signals used in medical imaging or diagnostic equipment. Variations in impedance can cause signal reflections and distortions, negatively impacting device accuracy and performance. This is typically achieved by tightly controlling the trace width, spacing, and dielectric properties of the PCB substrate. - Component Selection:
Choosing medical-grade components is critical. These components must meet specific biocompatibility standards, be highly reliable, and withstand sterilization procedures. This often includes selecting components with low outgassing and excellent temperature stability. Furthermore, considerations for end-of-life component availability and obsolescence planning are essential. - Layer Stack-up:
A well-defined layer stack-up is crucial for managing signal routing, power distribution, and thermal performance. The arrangement and type of layers significantly influence signal integrity, impedance control, and overall PCB performance. A balanced and well-thought-out stack-up also allows for adequate power and ground planes which contribute to the overall reliability of the board. - Thermal Management:
Medical devices often generate heat, and effective thermal management is critical for ensuring device reliability and patient safety. This involves careful component placement, the incorporation of heat sinks, and the selection of PCB materials with good thermal conductivity. In some cases, thermal simulation and modeling may be necessary for more complex designs. - Design for Manufacturability (DFM):
Working with your manufacturer early in the design process ensures the design is manufacturable and minimizes potential challenges during production. This can include design rule checks that consider the limitations of the specific manufacturer's fabrication capabilities and assembly processes.
Effective collaboration with your medical PCB manufacturer is paramount during the design phase. Their expertise in material selection, fabrication processes, and assembly techniques can help optimize your PCB design for performance, reliability, and cost-effectiveness. Clearly communicating design specifications and requirements to the manufacturer is crucial for ensuring alignment of expectations and for successful execution of the project.
Manufacturing Capabilities and Technology
Selecting a medical PCB manufacturer requires a thorough evaluation of their manufacturing capabilities and technological proficiency. These factors directly influence the quality, reliability, and suitability of the PCBs for medical applications, impacting performance and compliance with stringent medical device regulations. A capable manufacturer should possess advanced fabrication, assembly, and testing technologies to meet the diverse needs of medical device production.
| Capability/Technology | Description | Importance for Medical PCBs |
|---|---|---|
| Fabrication Technologies | Includes methods like multilayer lamination, micro-via drilling, and fine-line etching. | Crucial for creating complex, high-density PCBs required in advanced medical devices. Precision in these processes is critical for signal integrity and reliability. |
| Assembly Options | Surface mount technology (SMT) and through-hole technology (THT). | SMT is vital for miniaturized devices, while THT is better for robust components. Manufacturers should support both, offering flexibility to match design requirements. |
| Testing Methodologies | In-circuit testing (ICT), automated optical inspection (AOI), and functional testing. | Rigorous testing ensures defect-free PCBs and reliable performance, preventing costly failures in critical medical applications. |
| Material Handling | Process of storing, moving, and preparing materials. | Handling materials with care is important for keeping them contaminant-free and usable. |
| Volume Scalability | Capability to manage small prototype runs to large-scale production. | Medical device development requires flexibility in manufacturing volumes, and a manufacturer's scalability ensures smooth transitions from prototype to market. |
| Advanced Technologies | HDI (High-Density Interconnect) PCBs, embedded components, and flexible circuits. | Manufacturers should demonstrate proficiency in advanced techniques that enable miniaturization, complex functionalities, and innovative medical device designs. |
Quality Control and Testing Procedures
Rigorous quality control and testing are paramount for medical PCBs to guarantee their reliability and performance in critical medical applications. Medical PCB manufacturers employ a variety of testing protocols to ensure that each board meets stringent specifications and regulatory requirements, safeguarding patient safety and device functionality.
| Testing Method | Description | Purpose | Key Benefits |
|---|---|---|---|
| In-Circuit Testing (ICT) | Tests individual components and circuits on the assembled PCB using a 'bed of nails' fixture. | Detects manufacturing defects such as shorts, opens, and incorrect component values. | Early detection of faults, high accuracy, automated testing, ensures board functionality |
| Automated Optical Inspection (AOI) | Uses high-resolution cameras to capture images of the PCB and compare them to a known good standard. | Identifies visual defects such as solder joint issues, component placement errors, and missing parts. | Rapid inspection, non-contact method, identifies a wide range of defects, reduces manual inspection |
| Functional Testing | Simulates the operational environment of the PCB to verify its performance and functionality. | Verifies that the PCB functions as intended under specified conditions, testing its overall behavior. | Ensures the PCB meets functional specifications, validates design performance, provides end-to-end testing |
| X-Ray Inspection | Uses X-rays to penetrate the PCB and provide internal images. | Detects hidden defects such as solder voids, barrel cracks in through-hole vias, and component alignment under BGA packages. | Identifies internal defects not visible to the naked eye, critical for high-reliability boards, ensures internal integrity |
| Impedance Testing | Measures the characteristic impedance of PCB traces to ensure that signals travel correctly. | Verifies that trace impedances match design requirements, preventing signal reflection and ensuring signal integrity. | Crucial for high-speed and RF applications, ensures optimal performance, prevents signal degradation |
Frequently Asked Questions About Medical PCB Manufacturers
Selecting the right medical PCB manufacturer is a critical decision, influenced by factors ranging from regulatory compliance to specific manufacturing capabilities. This section addresses frequently asked questions to guide you through this selection process, ensuring your medical device PCBs meet the highest standards of quality and reliability.
- What criteria should I use to evaluate a medical PCB manufacturer?
When evaluating a medical PCB manufacturer, prioritize ISO 13485 certification, FDA registration, adherence to IPC standards, and a proven track record in medical device manufacturing. Assess their material selection process, manufacturing capabilities, testing protocols, and overall quality management system to ensure they meet the specific requirements of your project. - How do I find a reliable 'medical PCB manufacturer list'?
A reliable 'medical PCB manufacturer list' can be compiled by consulting industry associations, participating in trade shows, or searching online directories focused on medical device manufacturing. Verify the legitimacy of each listed manufacturer through certifications and customer reviews to confirm their expertise and capability to produce high-quality medical PCBs. Cross-referencing with multiple sources will provide a comprehensive perspective. - Who is the best PCB manufacturer for medical devices?
The 'best' PCB manufacturer for medical devices is subjective and dependent on the specific needs of your project. Consider manufacturers with specialized experience in medical PCB fabrication, robust quality control measures, and capabilities matching your design requirements. Focus on manufacturers with a strong reputation for compliance, reliability, and customer service within the medical industry, rather than simply the largest in scale. - Who is the largest manufacturer of medical supplies, and do they also make PCBs?
While several large companies dominate the medical supplies market, these companies do not necessarily manufacture PCBs. Medical device companies often outsource their PCB manufacturing to specialized PCB manufacturers. It's critical to seek out PCB manufacturers with a medical device specialization rather than assuming a large medical supplier also produces PCBs. - How do I verify if a medical PCB manufacturer is compliant with ISO 13485 standards?
To verify ISO 13485 compliance, request a copy of the manufacturer's certification document from a recognized certification body. Confirm the scope of the certification covers the manufacturing of medical PCBs. You may also consider conducting audits of their facilities, or review audit reports from other reputable clients to gain an independent confirmation of their compliance. - What level of testing should I expect from a medical PCB manufacturer?
Expect comprehensive testing, including in-circuit testing (ICT), automated optical inspection (AOI), and functional testing, at a minimum. A high-quality medical PCB manufacturer should provide test reports for each batch of PCBs to ensure they meet predefined quality and performance standards. More complex designs may require more tailored, comprehensive testing, as well as documentation that proves the validity of testing protocols and their results. - What are the typical lead times for medical PCBs, and what factors influence them?
Lead times for medical PCBs vary based on design complexity, material availability, and manufacturing volume. Expect longer lead times for highly specialized or complex PCBs, or those made with exotic materials. Clear communication, early design freeze and collaboration with the manufacturer are key to optimizing production and reducing potential delays. It's prudent to plan and understand these lead times early in the design cycle.
Cost Factors and Budgeting for Medical PCBs
The cost of medical PCBs is influenced by a multitude of factors, extending beyond simple material costs to encompass design complexity, production volume, and the rigorous testing required to meet medical device standards. Accurately budgeting for these elements is essential to maintain both the financial viability of a project and the required quality and reliability of the final product.
| Cost Factor | Description | Impact on Budget |
|---|---|---|
| Material Selection | Type and grade of materials used (e.g., FR-4, polyimide) | Higher-grade materials increase cost but ensure biocompatibility and longevity. |
| Design Complexity | Number of layers, component density, trace widths and spacing, impedance control requirements | Complex designs require more resources, thus increasing fabrication costs. |
| Manufacturing Volume | Quantity of PCBs ordered | Higher volume orders generally result in lower per-unit cost, utilizing economies of scale. |
| Testing Requirements | In-circuit testing (ICT), automated optical inspection (AOI), functional testing | Comprehensive testing ensures quality, though contributing to the overall manufacturing cost. |
| Certifications and Compliance | Adherence to ISO 13485, FDA registration, and other medical standards | Stringent quality and compliance requirements increase costs due to specific testing, auditing, and documentation needs. |
| Surface Finish | Gold, ENIG (Electroless Nickel Immersion Gold) or HASL surface finish | Finishes with better conductivity and longer shelf life will typically be more expensive. |
| Lead Time | Production time and expedited service | Faster lead times, and special handling can often result in increased expenses. |
| Component Sourcing | Component type, availability, and supplier costs. | Specialized medical components often command a higher cost. |
Comparing Medical PCB Manufacturers: A Checklist
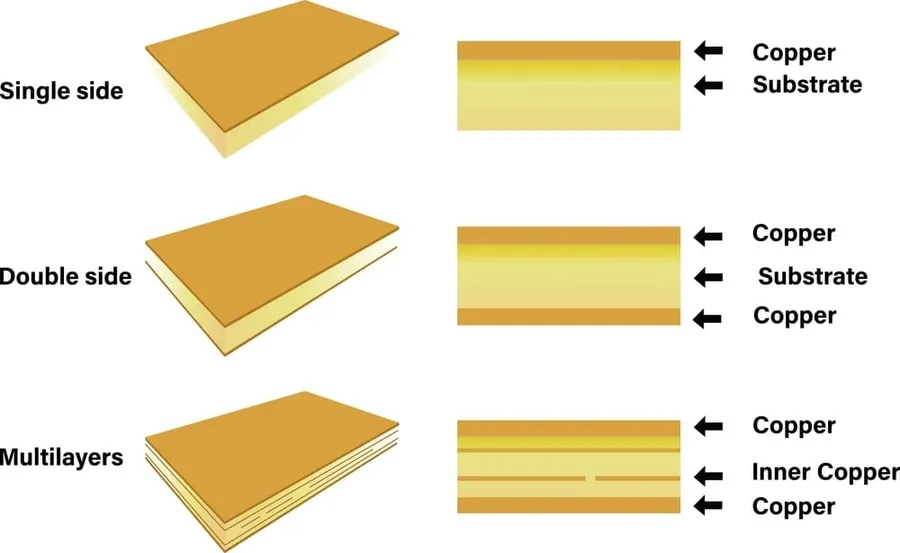
Selecting the right medical PCB manufacturer is a critical decision that directly impacts the quality and reliability of your medical device. This checklist provides a structured approach to evaluating potential manufacturers, ensuring you make an informed choice based on certifications, capabilities, experience, quality control, and cost.
| Evaluation Criteria | Checklist Items | Importance |
|---|---|---|
| Certifications and Compliance | ISO 13485 Certification, FDA Registration, IPC Standards Compliance, UL and IEC Compliance | Ensures adherence to industry-specific quality and regulatory standards, demonstrating a commitment to safety and performance. |
| Manufacturing Capabilities | Fabrication technology (e.g., multilayer, HDI), SMT and through-hole assembly, component placement accuracy, solder quality. | Verifies ability to produce PCBs that match your design specifications and handle specific complexity and volume requirements. |
| Experience and Expertise | Proven track record in medical PCB manufacturing, experience with medical device classifications (I, II, III), references and testimonials. | Demonstrates the manufacturer’s familiarity with the unique challenges and requirements of medical device PCBs. |
| Quality Control Measures | In-circuit testing (ICT), automated optical inspection (AOI), functional testing, reliability testing, material traceability. | Guarantees high-quality, defect-free PCBs through rigorous testing, ensuring consistent performance and longevity. |
| Cost and Budget | Transparent pricing structure, cost of materials, design complexity, manufacturing volume, testing costs | Helps you plan realistically without compromising on the quality and reliability of your medical PCB. |
| Communication and Support | Responsive project management, clear communication channels, engineering support during design, DFM analysis. | Ensures smooth collaboration, timely feedback, and a proactive approach to problem-solving. |
| Lead Times and Scalability | Ability to meet required production timelines, flexibility to scale up production as your demand changes, capacity for prototype to high volume manufacturing. | Ensures timely project completion and readiness for future demand. |
Choosing the right [medical pcb manufacturer] is a critical step in ensuring the safety and effectiveness of medical devices. By considering the factors discussed, such as certifications, material selection, design considerations, and quality control, manufacturers can make informed decisions that will guarantee high-quality PCBs that meet the stringent demands of the medical industry. As technology evolves, continuous evaluation of your manufacturing partners will be essential for maintaining a competitive edge and upholding patient safety standards, where the [medical pcb manufacturer] is an ongoing collaboration.
 AnyPCBA
AnyPCBA