Unveiling the Power of Electric Double Layer Capacitors (EDLCs): A Deep Dive
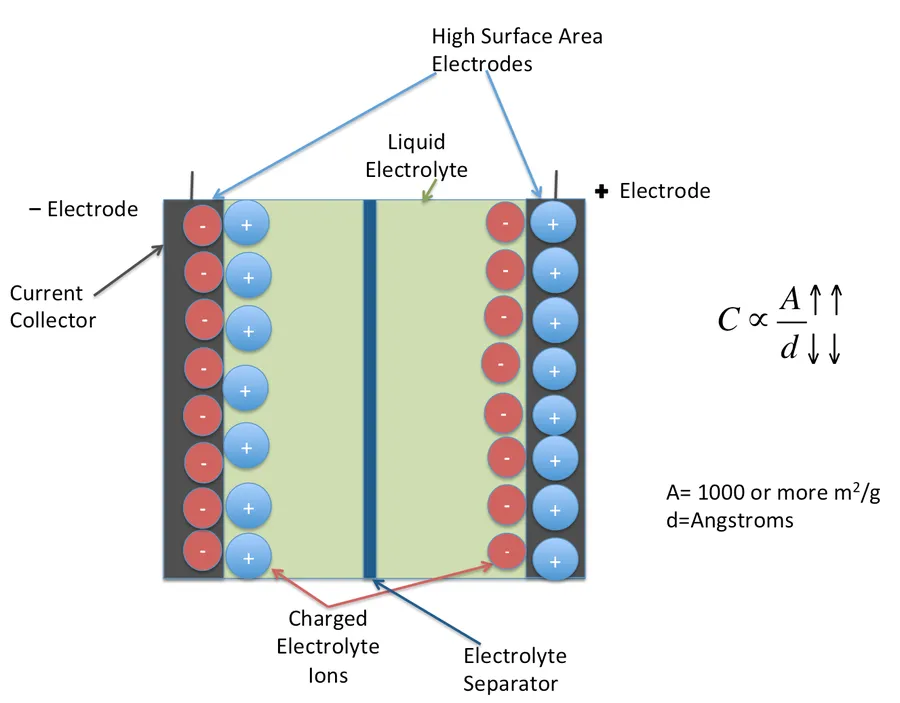
Imagine a world where energy storage is not just about batteries. The electric double layer capacitor, or EDLC, is revolutionizing how we think about energy storage. Unlike traditional capacitors that rely on a dielectric material, EDLCs utilize an electric double layer at the electrode-electrolyte interface to store energy. This innovative technology, bridging the gap between traditional capacitors and batteries, offers unique advantages in a wide range of applications from hybrid vehicles to portable electronics. This article will delve into the core concepts, advantages, disadvantages, and future of electric double layer capacitors.
The Core Principle: How Electric Double Layer Capacitors Work

Electric Double Layer Capacitors (EDLCs), also known as supercapacitors or ultracapacitors, store energy through the electrostatic accumulation of ions at the interface between an electrode material and an electrolyte. This phenomenon, known as electric double layer formation, is fundamentally different from the chemical reactions used in batteries and the dielectric polarization in traditional capacitors. Understanding this core principle is crucial to comprehending the unique performance characteristics of EDLCs.
The process involves two key layers: an inner Helmholtz layer, where ions from the electrolyte are adsorbed onto the electrode surface, and an outer diffuse layer, where a decreasing concentration of ions counterbalances the charge accumulated in the Helmholtz layer. This separation of charge results in a potential difference and is the basis for energy storage. This is a physical process, not a chemical one, so the energy storage of EDLC is very rapid and stable.
The ability of the EDLC to store charge is directly related to the surface area of the electrode material. High surface area materials such as activated carbon, carbon nanotubes, and graphene are often used to maximize charge storage. The electrolyte plays a vital role too, because the ions of the electrolyte create the electrical double layer.
| Component | Description | Role in EDLC |
|---|---|---|
| Electrode | Porous material with high surface area (e.g., activated carbon) | Provides the surface for ion accumulation |
| Electrolyte | Ionic conductor (e.g., aqueous, organic, ionic liquid) | Provides ions to form the double layer |
| Separator | Insulating material | Prevents electrical short circuit between electrodes |
| Current Collector | Conductive material (e.g., metal) | Facilitates the electron transport |
The electrochemical double layer capacitor doesn't rely on a chemical reaction but a physical one, which is the main difference between an EDL capacitor and a battery. Understanding this core principle allows for a deeper appreciation for EDLC's unique place in energy storage technologies. It also provides a basis for comparing EDL capacitors with traditional capacitors and batteries.
EDLC vs. Traditional Capacitors: Key Differences
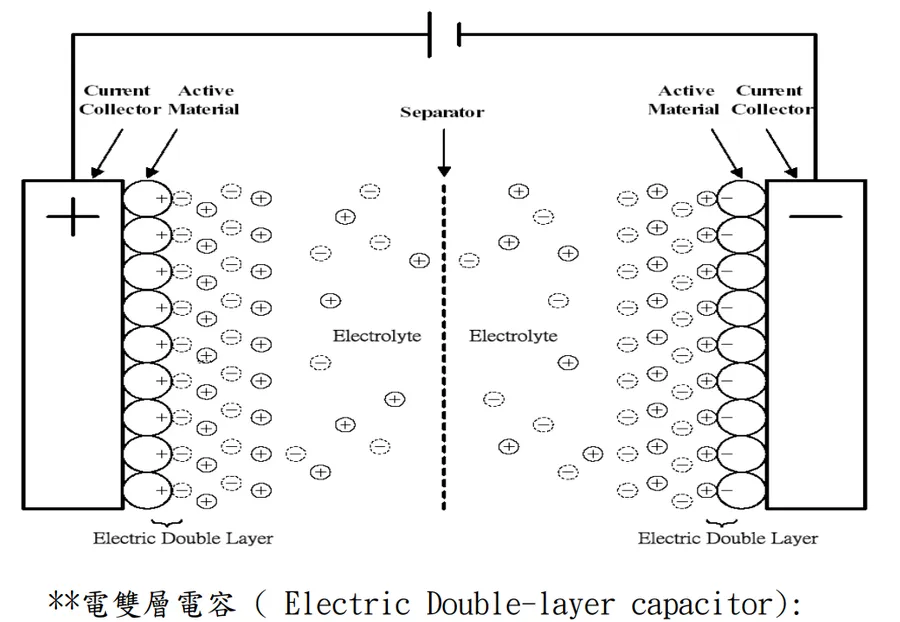
Electric Double Layer Capacitors (EDLCs) and traditional capacitors, while both serving as energy storage devices, diverge significantly in their structure, energy storage mechanisms, capacitance characteristics, and typical applications. These differences are crucial in determining their suitability for various technological implementations.
| Feature | Traditional Capacitor | Electric Double Layer Capacitor (EDLC) |
|---|---|---|
| Structure | Two conductive plates separated by a dielectric material | Two electrodes immersed in an electrolyte, with charge accumulation at the electrode-electrolyte interface |
| Energy Storage Mechanism | Electrostatic charge accumulation on the plates due to dielectric polarization | Electrostatic charge accumulation in the electric double layer at the electrode-electrolyte interface |
| Capacitance | Lower capacitance values | Higher capacitance values, resulting from the extremely small separation distances in the double layer |
| Energy Density | Lower energy density | Higher energy density compared to traditional capacitors, but lower than batteries |
| Power Density | Lower power density | Significantly higher power density than traditional capacitors and batteries |
| Charging/Discharging Rate | Slower charging and discharging rates | Very fast charging and discharging rates |
| Cycle Life | Moderate cycle life | Longer cycle life with minimal degradation |
| Applications | Filtering, timing circuits, energy storage in low power applications. | High power applications: regenerative braking, hybrid vehicles, backup power, portable electronics |
EDLC vs. Batteries: Where Do They Stand?
Electric Double Layer Capacitors (EDLCs) and batteries are both energy storage devices, but they operate on fundamentally different principles, leading to distinct performance characteristics and application suitability. Understanding these differences is crucial for selecting the optimal energy storage solution for a given application. EDLCs store energy electrostatically by accumulating ions at the electrode-electrolyte interface, while batteries rely on electrochemical reactions.
| Feature | EDLCs (Supercapacitors) | Batteries |
|---|---|---|
| Energy Storage Mechanism | Electrostatic accumulation of ions at the electrode-electrolyte interface. | Electrochemical reactions involving chemical transformations of active materials. |
| Energy Density | Lower, typically 5-10 Wh/kg. | Higher, typically 30-200+ Wh/kg (varies with technology). |
| Power Density | Significantly higher, typically 10 kW/kg or more. | Lower, typically 0.1-1 kW/kg. |
| Charge/Discharge Rate | Very fast, seconds to minutes. | Slower, typically hours. |
| Cycle Life | Very long, typically hundreds of thousands to millions of cycles. | Shorter, typically hundreds to thousands of cycles (varies with technology). |
| Operating Temperature | Wider operating temperature range. | More sensitive to temperature. |
| Voltage Characteristics | Voltage drops linearly during discharge. | Relatively constant voltage discharge. |
| Environmental Impact | Generally more environmentally friendly due to material composition and absence of chemical reactions. | Can have a higher environmental impact due to toxic materials and chemical waste. |
| Cost | Higher per unit of energy storage, but lower over a long life cycle. | Lower upfront cost, but may have a shorter overall lifespan. |
| Applications | Applications requiring high power delivery for short durations (e.g., regenerative braking, power assist). | Applications requiring long-term energy storage (e.g., electric vehicles, portable electronics). |
Types of Electric Double Layer Capacitors: Materials and Configurations
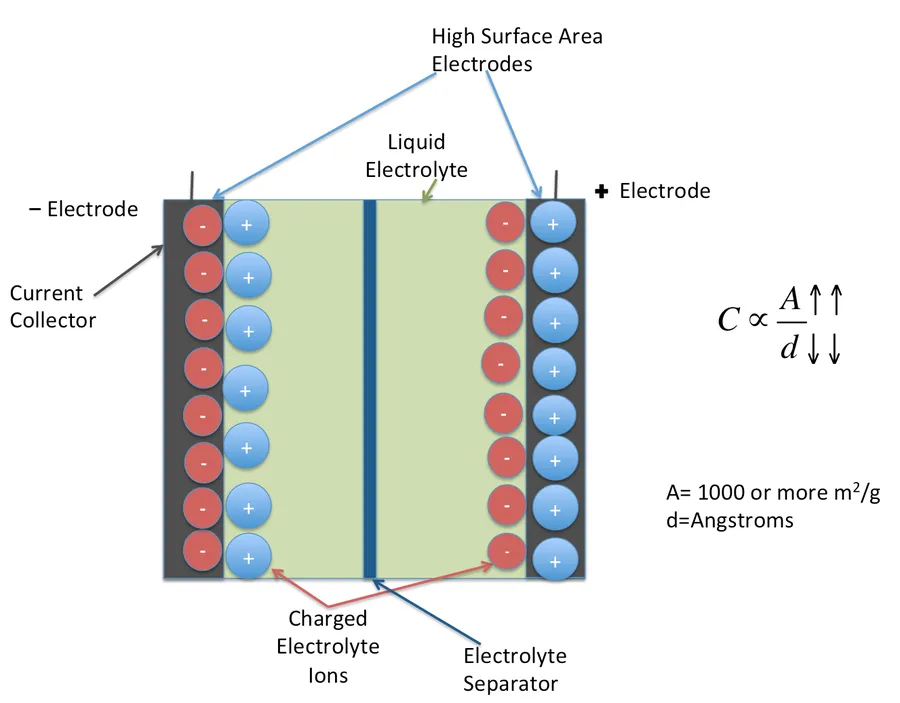
Electric Double Layer Capacitors (EDLCs) exhibit diverse characteristics depending on the materials employed for electrodes and electrolytes, as well as their configuration. Understanding these variations is crucial for tailoring EDLCs to specific applications, optimizing performance, and cost-effectiveness. The selection of materials and configuration directly influences the EDLC's capacitance, voltage window, and overall performance.
| Component | Material Type | Description | Advantages | Disadvantages |
|---|---|---|---|---|
| Electrode Material | Activated Carbon | Porous carbon material with high surface area. | Low cost, high availability, good electrical conductivity. | Relatively lower energy density compared to other materials. |
| Electrode Material | Carbon Nanotubes (CNTs) | One-dimensional cylindrical nanostructures with excellent electrical conductivity. | High conductivity, excellent mechanical properties, good charge transfer. | Higher cost, challenges in large-scale production, tendency to agglomerate. |
| Electrode Material | Graphene | Two-dimensional sheet of carbon atoms with exceptional electrical conductivity and surface area. | High surface area, exceptional conductivity, good electrochemical stability. | Higher cost, challenges in mass production, tendency to restack. |
| Electrolyte | Aqueous Electrolytes | Water-based electrolytes, often with dissolved salts. | High ionic conductivity, low cost, environmentally friendly. | Limited voltage window, potential for electrochemical decomposition. |
| Electrolyte | Organic Electrolytes | Non-aqueous electrolytes based on organic solvents. | Higher voltage window, good stability. | Higher cost, lower ionic conductivity than aqueous electrolytes, environmental concerns due to flammability and toxicity. |
| Electrolyte | Ionic Liquids | Salts that are liquid at room temperature. | High voltage window, good electrochemical and thermal stability, negligible vapor pressure. | Higher cost, lower ionic conductivity than aqueous electrolytes. |
| Configuration | Symmetrical EDLC | Both electrodes use the same material. | Simpler design and manufacturing, cost-effective. | Limited energy density and voltage. |
| Configuration | Asymmetrical EDLC | Electrodes with different materials are used to expand the voltage window and energy density. | Higher energy density and voltage, flexible design parameters. | More complex design, require careful material matching and control, cost-effective. |
Advantages of Electric Double Layer Capacitors: Why Choose EDLCs?
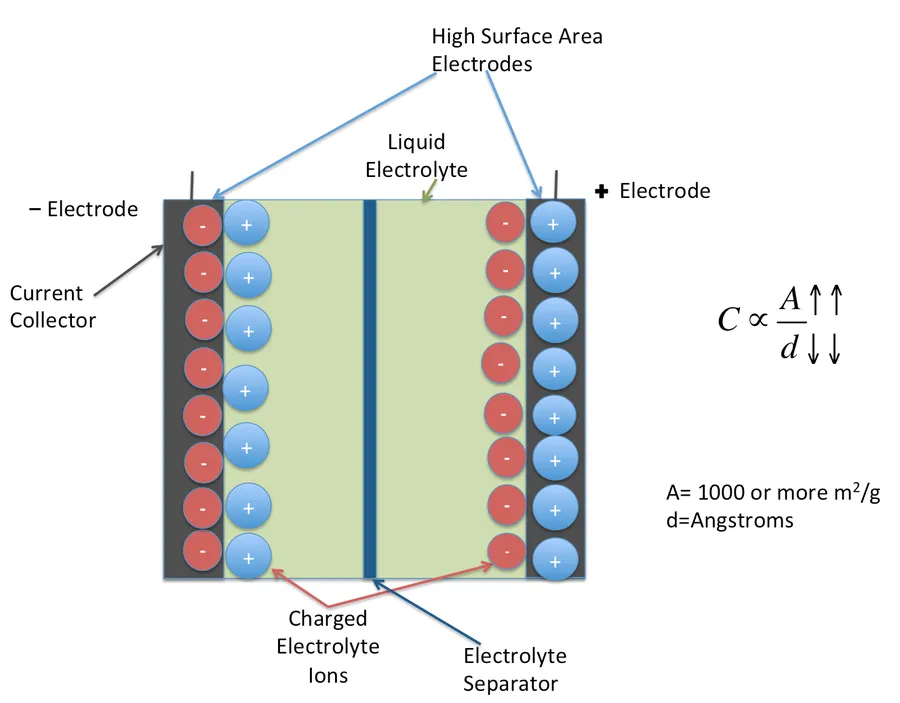
Electric Double Layer Capacitors (EDLCs) offer several compelling advantages, making them a preferred choice for specific applications where high power delivery, rapid charge-discharge cycles, and long operational lifespan are critical. These benefits stem directly from the fundamental charge storage mechanism of EDLCs, which relies on the physical accumulation of ions at the electrode-electrolyte interface rather than chemical transformations.
- High Power Density
EDLCs boast exceptionally high power density compared to batteries. This translates to a rapid delivery of electrical energy, crucial for applications requiring bursts of power. The physical charge storage mechanism, devoid of chemical reactions, facilitates faster ion movement and therefore, higher power output. - Fast Charging and Discharging Rates
The charge/discharge rates of EDLCs are significantly higher than those of batteries, typically by orders of magnitude. This ability to rapidly accept and deliver charge is advantageous in applications like regenerative braking and pulsed power devices. The absence of phase transitions during charging and discharging contribute to this speed. - Long Cycle Life
EDLCs demonstrate excellent cycle life, often exceeding hundreds of thousands or even millions of charge/discharge cycles. This durability is attributed to the non-chemical nature of charge storage, reducing degradation of materials and ensuring long-term stable performance. This contrasts with batteries where chemical reactions lead to material degradation over time. - Wide Operating Temperature Range
EDLCs can operate efficiently across a broad range of temperatures, making them suitable for challenging environments. The stability of the electric double layer is less affected by temperature changes compared to the temperature-sensitive chemical reactions in batteries, enabling consistent performance in both hot and cold conditions. - Environmental Friendliness
EDLCs are generally considered environmentally benign, as they do not typically contain toxic heavy metals, and their raw materials are more abundant. The non-chemical energy storage mechanism results in less harmful waste upon disposal compared to batteries. The materials used in EDLCs such as carbon and some electrolytes are less environmentally concerning than some of the heavy metals found in batteries.
Limitations of Electric Double Layer Capacitors: Addressing the Drawbacks
Despite their numerous advantages, electric double-layer capacitors (EDLCs) are not without limitations. These drawbacks primarily stem from the fundamental mechanism of energy storage at the electrode-electrolyte interface. Addressing these limitations is the focus of ongoing research and development efforts to broaden the applicability of EDLCs.
- Lower Energy Density Compared to Batteries
EDLCs store energy electrostatically, accumulating ions at the electrode surface. This contrasts with batteries that store energy via chemical reactions. Consequently, EDLCs generally have a lower energy density (typically 5-10 Wh/kg) compared to electrochemical batteries which can achieve 50-200 Wh/kg. This is a primary limitation that restricts the amount of energy stored in a given size or weight for EDLCs. - Voltage Drop During Discharge
Due to the capacitive nature of charge storage, the voltage across an EDLC decreases linearly with the discharge, unlike batteries, which maintain a more stable voltage for most of the discharge cycle. This voltage drop may be problematic in applications requiring a steady voltage supply. - Higher Cost Compared to Traditional Capacitors
The manufacturing of EDLCs can be more expensive than traditional capacitors, mainly due to the specialized materials and fabrication processes needed to create large surface area electrodes and optimized electrolytes. Although costs are decreasing with advances in manufacturing scale, it remains a limitation in price-sensitive applications. - Performance Degradation Over Time
EDLCs can exhibit some performance degradation with repeated use, due to factors like electrolyte decomposition, electrode material oxidation, and contact resistance changes. This means that there is a small decrease in capacity and an increase in equivalent series resistance (ESR) with cycling.
Ongoing Research is focused on mitigating these issues. This involves advanced material research and exploration of innovative device architectures. Some of these research directions include:
- Advanced Materials for Higher Energy Density
Research into advanced electrode materials with higher surface area and capacitance, like graphene and MXenes, aims to achieve greater energy density without compromising power density. These materials can improve both the energy density and capacitance. - Electrolyte Improvements for Extended Voltage Window
Efforts are being made to develop electrolytes that are stable at higher operating voltages which can increase the overall energy storage capability. Ionic liquids and solid-state electrolytes are being developed for improved performance. - Hybrid Devices to Mitigate Voltage Drop
The combination of EDLCs with battery-like material is also being considered to create ‘hybrid’ devices with a more stable voltage window. This design seeks to optimize energy and power densities simultaneously.
Applications of EDLCs: Powering the Future
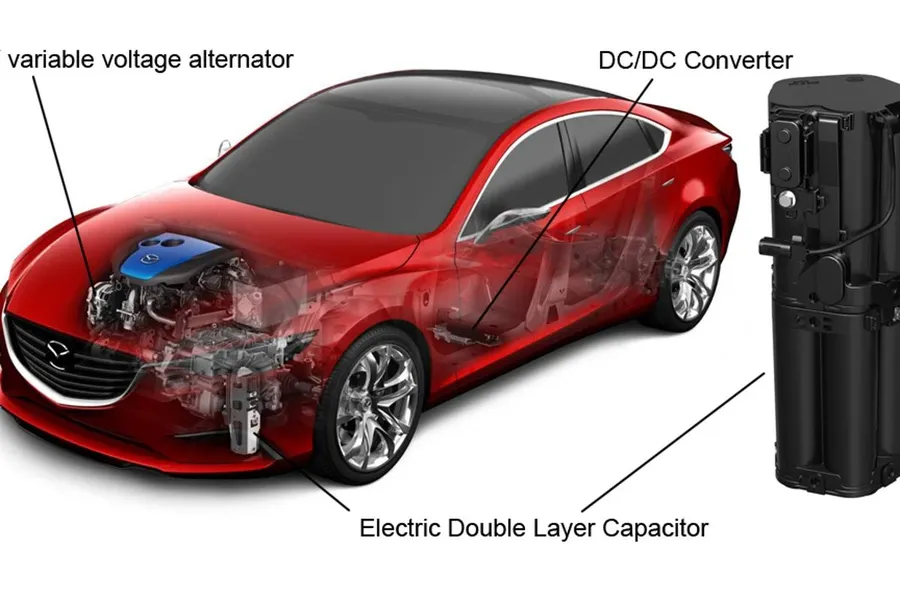
Electric Double Layer Capacitors (EDLCs), with their unique ability to rapidly store and release energy, are finding increasingly diverse applications across various sectors. Their high power density, long cycle life, and fast charge/discharge capabilities make them ideal for situations where bursts of energy are needed or frequent cycling is required, areas where traditional batteries struggle.
- Hybrid and Electric Vehicles (HEVs/EVs)
EDLCs are used in regenerative braking systems to capture and reuse energy during deceleration, improving overall vehicle efficiency. They are also used for power boosting during acceleration and to stabilize voltage fluctuations, extending battery life and enhancing vehicle performance. - Regenerative Braking Systems
In applications beyond vehicles, EDLCs capture kinetic energy during braking or deceleration in various machinery, converting it into electrical energy for later use. This is essential for improving energy efficiency in material handling equipment, cranes, and elevators. - Portable Electronics
EDLCs are suitable for powering devices requiring quick charging and discharging, such as portable tools, flashlights, wearable electronics, and digital cameras. They enable rapid power delivery and extend the operational lifespan of these devices. - Renewable Energy Storage
EDLCs can be used in conjunction with renewable energy sources like solar and wind power to manage fluctuating power outputs. They can store excess energy generated by these sources and deliver it when demand is high, thereby smoothing out energy supply and improving the reliability of renewable energy systems. - Backup Power Systems
EDLCs can provide uninterrupted power supply during grid failures or sudden power outages. Their ability to rapidly deliver high power makes them ideal for protecting critical equipment, industrial control systems, and computer hardware where even brief power interruptions can be detrimental. - Public Transportation
EDLCs have been incorporated into trams, buses, and trains, especially those operating on fixed routes, where frequent stops and starts benefit from regenerative braking and rapid power delivery. This results in significant energy savings and improved operational efficiency. - Industrial Equipment
EDLCs are used in various industrial applications, such as automated guided vehicles (AGVs), forklifts, and robotics, where they enable fast charging, high power delivery, and long cycle life. This boosts overall productivity and reliability.
Frequently Asked Questions About Electric Double Layer Capacitors
This section addresses common queries regarding Electric Double Layer Capacitors (EDLCs), providing clear and concise answers to enhance understanding of their operational principles, advantages, and applications.
- What is an Electric Double Layer Capacitor (EDLC)?
An Electric Double Layer Capacitor (EDLC), also known as a supercapacitor or ultracapacitor, is an electrochemical energy storage device that stores energy electrostatically by accumulating ions at the interface between an electrode and an electrolyte, forming an electrical double layer. Unlike batteries, which store energy through chemical reactions, EDLCs store energy physically, enabling rapid charging and discharging. - What is the Electrical Double Layer?
The electrical double layer is the fundamental mechanism of energy storage in EDLCs. It consists of two layers: an inner Helmholtz layer, where ions are specifically adsorbed onto the electrode surface, and a diffuse Gouy-Chapman layer, where ions are less strongly bound and more freely distributed within the electrolyte. This charge separation is what stores the energy. - How does an EDLC differ from a traditional capacitor?
Traditional capacitors store energy by accumulating electrons on conductive plates separated by a dielectric material. EDLCs, conversely, store energy by the accumulation of ions at an electrode-electrolyte interface, creating the double layer. EDLCs achieve much higher energy density and capacitance than traditional capacitors due to the extremely small separation distance of the double layer, resulting in significantly higher surface areas. - How does an EDLC differ from a battery?
While both EDLCs and batteries store energy, they differ significantly in their mechanisms and characteristics. Batteries store energy via chemical reactions, resulting in higher energy density but slower charging/discharging rates and limited cycle life. EDLCs use electrostatic charge accumulation, enabling very high power density, extremely fast charging/discharging, and an exceptionally long cycle life. However, EDLCs typically have a lower energy density than batteries. - What is the operating principle of an EDLC?
The principle of EDLC operation revolves around the formation of the electrical double layer at the interface between an electrode material and an electrolyte. When a voltage is applied, ions from the electrolyte migrate to the electrode surfaces, forming layers of opposite charge. This charge separation stores energy electrostatically. When the external voltage is removed or reversed, ions move back into the electrolyte, releasing the stored energy. - What materials are commonly used in EDLCs?
Common electrode materials in EDLCs include activated carbon, carbon nanotubes, and graphene, chosen for their high surface area and conductivity. Electrolytes can be aqueous, organic, or ionic liquids, selected for their ionic conductivity and electrochemical stability. These materials directly affect performance, cost, and the operational temperature range of EDLCs. - What are the key advantages of using EDLCs?
EDLCs offer several benefits including very high power density (rapid charging and discharging), long cycle life (hundreds of thousands to millions of cycles), a wide operating temperature range, and are considered more environmentally friendly due to the absence of chemical transformations during energy storage. These advantages make them suitable for applications requiring rapid energy delivery and repeated charging/discharging.
The Future of Electric Double Layer Capacitors: Trends and Innovations
The trajectory of Electric Double Layer Capacitors (EDLCs) is marked by continuous advancements in materials science, performance enhancement, and cost reduction, paving the way for their expanded application across diverse sectors. This evolution is driven by the demand for high-performing, sustainable energy storage solutions.
- Advanced Material Research
Research is focusing on novel electrode materials such as graphene and MXenes, which offer superior conductivity, higher surface areas, and improved electrochemical stability compared to traditional activated carbon. These advanced materials aim to maximize charge storage capacity and energy density. - Electrolyte Innovations
The development of new electrolyte materials, including ionic liquids and solid-state electrolytes, are being explored to enhance the operating voltage, ionic conductivity, and safety of EDLCs. Solid-state electrolytes, in particular, hold promise for eliminating leakage risks and improving the lifespan of the device. - Performance Optimization
Future development will concentrate on improving the energy density of EDLCs without compromising their unique attributes, such as high power density and long cycle life. Strategies such as optimizing the electrode-electrolyte interface and device configurations are under investigation to boost overall efficiency. - Cost Reduction
Efforts are being made to reduce the overall manufacturing costs of EDLCs through innovative production methods and the use of more accessible raw materials. This focus on cost-effectiveness is crucial for broader market penetration and commercial scalability. - Expansion of Application Areas
EDLCs are progressively finding applications beyond existing uses, including grid-scale energy storage, high-performance computing, and advanced portable electronics. These applications exploit their excellent power delivery and rapid charging capabilities. - Integration with Renewable Energy Systems
Future trends include the increased integration of EDLCs within renewable energy systems, providing a crucial interface for bridging the gap between intermittent energy production from solar and wind and the demands of the electrical grid. EDLCs can contribute to grid stabilization and improve the overall efficiency of renewable energy infrastructure.
The electric double layer capacitor represents a critical advancement in energy storage technology. By harnessing the power of the electric double layer, EDLCs offer high power capabilities and long cycle life. While there are limitations, ongoing research and development are continuously enhancing their performance. As the need for efficient and sustainable energy storage solutions grows, EDLCs are poised to play a vital role in shaping the future of various industries and applications, paving the way for a greener and more technologically advanced tomorrow.
 AnyPCBA
AnyPCBA